Research project objectives
Catalysis is extremely important for clean energy productions as well as key parameter for further development of chemical and pharmaceutical industries. TiO2 is the most commonly used photo-catalyst for the conversion of solar radiation into chemical bonds. While being very effective catalytic material, TiO2 requires excitation in the UV-regime due to its wide band gap energy (ca. 3.2 eV). The common strategy for improving visible light absorption is via structural modification/doping aimed at diminishing the TiO2 band gap energy.
The project aims at studying TiO2 surface/bulk electronic structure modified by carbon, nitrogen and sulfur doping. Our scientific approach will combine in-situ resonant X-ray emission spectroscopy (RXES) and theoretical approaches (FEFF and FDMNES calculations) to determine lowest occupied and highest unoccupied Ti electronic states at material's working conditios. This knowledge is necessary to understand why the doping-induced effect of increased visible light absorptions, in many cases does not translate to improved material's catalytic activity. Since the chemical effciency is determined by number of surface states available for reactions, effect of doping on the surface electronic structure have to be thus determined.
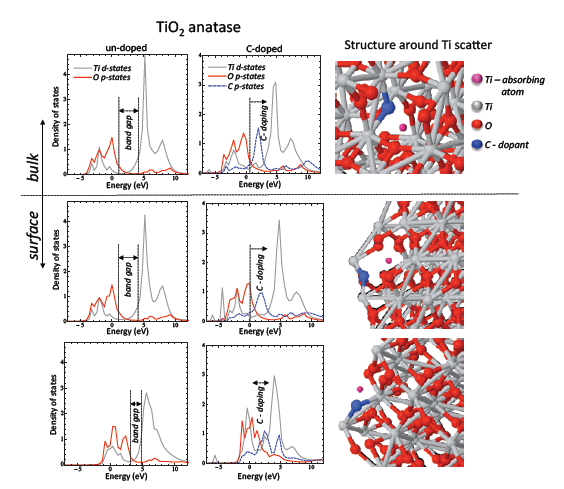
Figure 1. FEFF9.0 calculations for 1nm TiO2 nanoparticle. left) calculated TiO2 anatase density of states for bulk and surface states, middle) bulk and surface density od states for C-doped TiO2. As shown, C-doping induce band gap narrowing from side of valence electronic states, right) positions of Ti-scatter and surrounding atoms within TiO2 anatase structure.
Research project methodology
Unique properties of hard X-rays provide wide range of applications to study the photocatalytic materials, not only in steady-state environment, but also at in-situ and material's operating conditions. In the proposed project we will combine experimental and theoretical approaches to probe the electronic structure of TiO2-doped ultra-thin films. The project relies on last years of our development in application of resonant X-ray emission spectroscopy to study electronic properties of matter. In particular we willemployestabliszed by Szlachetko & Sa methodology for resonant X-ray emission spectroscopy data interpretation aiming at rational design of photo-catalytic materials.
The electronic structure of TiO2 and TiO2-doped samples will be probed by in-situ RXES spectroscopy. The experiments will be performed around K-absorption edge of Ti and by detection of core-to-core (Ka & Kß) and valence-to-core X-ray emission signals. Measurements will provide a complete electronic picture of electronic states around Ti atoms. Combination of in-situ RXES spectroscopy with grazing incidence geometry will allow disentagling surface and bulk electronic states at material's working conditions. In order to obtain full information about studied materials, the RXES measurements will be complemented with laboratory experimentals (like X-ray diffraction, XPS) at Institute Of Physics, UJK, Kielce as well as optical experiments in Laboratory for Spectroscopic Imaging (in collaboration with prof. W. Kwiatek). The experimental RXES data will be evaluated with theoretical computations to determine contributions of different electronic orbitals to measured signals. We will use available codes for electronic structure and density of states calculations. Comparison of measured data and calculated spectra wil give detailed information about accuracy of muffin-tin and finite difference computational methods to studied TiO2-based systems.
Figure 2. Kß-RXES plane of TiO2 anatase measured for Ti around K-absorption edge. The main detected apectroscopic features are labelled on the RXES plane. On top, the non-resonant Kß and valence-to-core X-ray emission spectrum is shown which was recorded for incidence X-ray energies above K-shell ionization threshold. Right) Extracted from RXES plane valence and conduction electronic structure is compared to density od states calculations.
Expected impact of the research project on the development of science, civilization
and society
Fujishima and Honda published the first significant breakthrough converting light into chemical ebergy in 1972. They reported the electrochemical photolysis of water assisted by a semiconductor under UV-A radiation. Most of developments are based on large band gap semiconductors (>3 eV), such as TiO2. This is, however, hampered by the need for UV-A (300-400nm) irradiation to induce charge separation. UV-A accounts for only 4% of the solar spectrum. Nevertheless, TiO2 remains indisputablynthe best performing photo-catalyst to date, and several efforts are been made to improve visible light absorption, including TiO2 band gap manipulation generally by doping with elements, such as N, C and S.
The present project aims at understanding why, reducing the band-gap energy of TiO2 by doping, which leads to increased visible light absorption, deos not affect or even often diminish material's overall catalytic activity. We intend to correlate surface reactivity with electronic structure changes in TiO2 induced by doping. Disentagling surface and bulk contributions will allow for deep insight understanding of photo-catalytic properties of doped-TiO2 and in consequence will help in future development of chemical or pharmaceutical applications.
